Rapid amyloid clearance in hours: what this 2025 mouse study shows us
Could the fastest way to treat Alzheimer’s disease be not to attack plaques in the brain, but to restore the brain’s clearance plumbing?
Alzheimer’s disease has long been one of medicine’s most stubborn challenges. For decades, scientists have approached this battle on two fronts: stopping the buildup of amyloid plaques in the brain, and getting drugs past the brain’s ultra-strict protective barrier, the Blood-Brain Barrier (BBB).
A recent 2025 paper in Signal Transduction and Targeted Therapy reported a striking finding in an Alzheimer’s mouse model: using nanotechnology, they were able to half brain amyloid within 2 hours, and importantly, restored spatial learning and memory in the mice, comparable to their normal peers [1].
Whilst an interesting breakthrough, it is also, currently unambiguously preclinical.
What’s different: repair the barrier, do not just try to cross it
Amyloid β and tau proteins are central to the pathological process in Alzheimer’s disease. Amyloid β tends to accumulate first and may help trigger the disease cascade, while tau tracks more closely with neurodegeneration and symptom severity in Alzheimer’s disease.
The BBB is a barrier that separates blood flow from the brain, protecting the brain from any potential harms due to blood-borne risk such as pathogens or toxins. The BBB does more than block toxins and pathogens though, it also helps keep the brain clean by transporting waste products like amyloid β out of the brain and into the bloodstream for disposal.
Many Alzheimer’s drug developments focus on the BBB as a wall that must be penetrated for the therapy to work. This paper suggests treating the blood brain barrier as a failing organ you might be able to fix. Instead of targeting neurones, their approach employed nanotechnology to restore the function of the BBB.
Supporting the theory of the BBB as a potential therapeutic target, human studies suggest signs of capillary damage and breakdown of the blood brain barrier can show up early in cognitive decline, and can relate to cognitive outcomes even when amyloid and tau biomarkers do not fully explain the picture [2].
The target: LRP1, a key amyloid export route
The authors focused on the low-density lipoprotein receptor-related protein 1 (LRP1), a receptor expressed on the blood brain barrier. It sits on the blood-brain barrier, recognises amyloid β and shuttles it out of the brain and into the bloodstream where it can be safely removed.
In Alzheimer’s patients, this pump breaks down. There are fewer LRP1 receptors expressed on the BBB and the remaining receptors get bound up by sticky amyloid beta, which leads to many of them being destroyed.
The team compared mice genetically altered to develop Alzheimer’s-like amyloid build up with normal mice used as controls. They created a special type of nanomedicine called LRP1-targeted polymersomes. These tiny, synthetic spheres are engineered to be the perfect size and shape to latch onto the LRP1 receptors. Here, they signal to the cells to stop destroying the LRP1 pumps and instead send them back to the cell surface to do their job and clear amyloid β.
The Results
Rapid Cleaning: Within just 2 hours of injection, the nanoparticles reduced the levels of toxic amyloid β in the brain by nearly 45%.
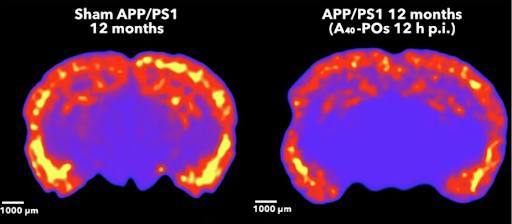
Figure 1: imaging showing a colour map of amyloid in a mouse brain slice, with the treated group (right) showing less high intensity signal 12 hours after treatment than the untreated control group (left).
Systemic Flush: At the same time, amyloid levels in the blood shot up by 8-fold, proving the toxins were being successfully pumped out of the brain.
Memory Restored: Most importantly, the mice regained their memory. In cognitive tests, the treated Alzheimer's mice performed just as well as healthy, young mice.
Long-Term Benefit: The cognitive improvements didn't just last for a few days, they persisted for 6 months after treatment.
Conclusions
This study represents a shift in how we can think about the design of drugs. Usually, nanoparticles are just envelopes used to deliver a drug into the body. Here, the nanoparticle is the drug. Its physical structure, how it interacts with the cell surface, is what triggers the therapeutic action.
By focusing on repairing the body's natural machinery rather than introducing foreign chemicals to attack the disease, this approach offers a novel way to treat neurodegenerative diseases.
Whilst encouraging, it’s important to note that this is still a long way off successful human clinical trials.
FAQs
Does this study show Alzheimer’s can be reversed in humans?
No.
It shows rapid amyloid reduction and improved performance on mouse cognitive tasks in an Alzheimer’s mouse model after a short dosing course.
What is the most important novelty here?
The mechanism: targeting the blood-brain barrier to shift amyloid clearance by preserving or restoring LRP1 function.
How big was the amyloid effect?
By ELISA at 2 hours: brain amyloid β fell from 8603.6 to 4236.3 ng/ml (~45%), with plasma rising from 85.3 to 673.5 ng/ml (8 times).
Further Reading
[1] J. Chen et al., ‘Rapid amyloid-β clearance and cognitive recovery through multivalent modulation of blood–brain barrier transport’, Signal Transduct. Target. Ther., vol. 10, no. 1, p. 331, Oct. 2025, doi: 10.1038/s41392-025-02426-1.
[2]D. A. Nation et al., ‘Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction’, Nat. Med., vol. 25, no. 2, pp. 270–276, Feb. 2019, doi: 10.1038/s41591-018-0297-y.